[6][8] For comparison, on top of Mount Everest, at 8,848m (29,029ft) elevation, the pressure is about 34kPa (255Torr)[9] and the boiling point of water is 71C (160F). Credit: Watch Lindsey Ogle livestreams, replays, highlights, and download the games You'll get the latest updates on this topic in your browser notifications. Returning to camp after losing her closest ally, NBA star Cliff Robinson, Ogle got into a heated argument with fellow castaway Trish Hegarty. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. Youre in the right place! Edit. 6131 views I told him, I don't feel comfortable with this. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and
You could just kinda tell by the energy of what was going on: There's gonna be some mix-ups, there's gonna be some twists, there's gonna be some turns.  The following link will open in a new window. Even the pole challenge. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. Providing global relocations solutions, storage and warehousing platforms and destruction plans. And a lot of people are like, You're blaming it on your daughter. That's still what I'm feeling like, Oh! I don't know. When the molecular size becomes that of a macromolecule, polymer, or otherwise very large, the compound often decomposes at high temperature before the boiling point is reached. Take an example such as Mount Elbert, Colorado, the highest peak of the Rocky Mountains and the highest elevation point in the United States. Someone might think, Oh, that Lindsey. All my love to you.
The following link will open in a new window. Even the pole challenge. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. Providing global relocations solutions, storage and warehousing platforms and destruction plans. And a lot of people are like, You're blaming it on your daughter. That's still what I'm feeling like, Oh! I don't know. When the molecular size becomes that of a macromolecule, polymer, or otherwise very large, the compound often decomposes at high temperature before the boiling point is reached. Take an example such as Mount Elbert, Colorado, the highest peak of the Rocky Mountains and the highest elevation point in the United States. Someone might think, Oh, that Lindsey. All my love to you.
What is the molar mass of the compound? Him and I talked for quite a long time and a lot of people are like, Ugh. 2,624 likes. Lindsey: No! 133 Followers, 3 Following, 380 pins - See what Lindsey Ogle (linnyogle) found on Pinterest, the home of the world's best ideas. David Samson, Jazmine Sullivans Heaux Tales Reveres Women With Grace And Self-Love, The Indie Rockers To Watch Out For In 2021, Coming 2 America Is A Rare Comedy Sequel That Does Justice To The Original, With Oscar-Worthy Costume Design As The Cherry On Top, The Rundown: Desus And Mero Are The Best And They Did Something Really Cool This Week, Jared Hess And Tyler Measom On Exploring Mormon Eccentricity In Murder Among The Mormons, The Reddit-GameStop Saga Is A Billions Episode Happening In Real-Time, Indigenous Comedians Speak About The Importance Of Listening To Native Voices, Indigenous Representation Broke Into The Mainstream In 2020, Author/Historian Thomas Frank On Why The Democratic Party Needs To Reclaim Populism From Republicans, The Essential Hot Sauces To Make 2021 Pure Fire, Travel Pros Share How They Hope To See Travel Change, Post-Pandemic, A Review Of Pizza Huts New Detroit Style Pizza, Were Picking The Coolest-Looking Bottles Of Booze On Earth, MyCover: Arike Ogunbowale Is Redefining What It Means To Be A Superstar, Tony Hawk Still Embodies Skateboard Culture, From Pro Skater 1+2 To Everyday Life, Zach LaVines All-Star Ascension Has The Bulls In The Playoff Hunt, Talib Kweli & DJ Clark Kent Talk Jay-Z vs. Biggie, Superman Crew, & Sneakers, Ruccis Heartfelt UPROXX Sessions Performance Implores You To Believe In Me, BRS Kash, DDG, And Toosii React To Adina Howards Freak Like Me Video, Obsessed: Godzilla Vs. Kong, Cruella, And More Spring Blockbusters We Cant Wait To Watch. Susan quit because Richard Hatch rubbed against her. Most volatile compounds (anywhere near ambient temperatures) go through an intermediate liquid phase while warming up from a solid phase to eventually transform to a vapor phase. All rights reserved. I don't even want to tell you!
It's Survivor. You never know what's gonna happen. Take an example such as Mount Elbert, Colorado, the highest peak of the Rocky Mountains and the highest elevation point in the United States. Kierans bookClimbing the Wallsan exploration of the mental health benefits of climbing, mountaineering, and the great outdoorsis scheduled for release by Simon & Schuster in April 2021. Your boil-water notice queries answered:Can I use my dishwasher during a boil-water notice? As the altitude increases the boiling point of water decreases. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. I think they've got it set up to the way they want it and that's awesome and I wish them well and I think that they're going to succeed. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg.
I started sweating. ThoughtCo. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). Because of these two phenomena, the liquid range of a solvent is increased in the presence of a solute. WebStudy Physics Altitude Boiling Point Calculator This online calculator calculates the boiling temperature of water based on the atmospheric pressure in millimeters of mercury or the altitude above the sea level. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. Survivor isn't a show for quitters and yet many players have quit on Survivor over 28 seasons.  It is a phenomenon that happens for all solutes in all solutions, even in ideal solutions, and does not depend on any specific solutesolvent interactions. But putting yourself out there? I can't believe you. Jeff's a pretty honest guy. Timing is key At high altitudes, cooking times are longer, even though water boils faster How to Pack a Backpack Are You Doing it Right? It depends on where youre doing the boiling. Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90. Note! And I happen to be on the losing side of it, but it's what you do with the game that you've gotten, even if it was five seconds or not. The boiling point cannot be reduced below the triple point. Look! You know?
It is a phenomenon that happens for all solutes in all solutions, even in ideal solutions, and does not depend on any specific solutesolvent interactions. But putting yourself out there? I can't believe you. Jeff's a pretty honest guy. Timing is key At high altitudes, cooking times are longer, even though water boils faster How to Pack a Backpack Are You Doing it Right? It depends on where youre doing the boiling. Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90. Note! And I happen to be on the losing side of it, but it's what you do with the game that you've gotten, even if it was five seconds or not. The boiling point cannot be reduced below the triple point. Look! You know?
Thats why instead we have the hard facts for you on the science behind the temperature of boiled water, how fast it will boil, and whether a lid on your pot or some salt in your water will speed up the process. I usually get along with people, but Trish just rubbed me the wrong way. 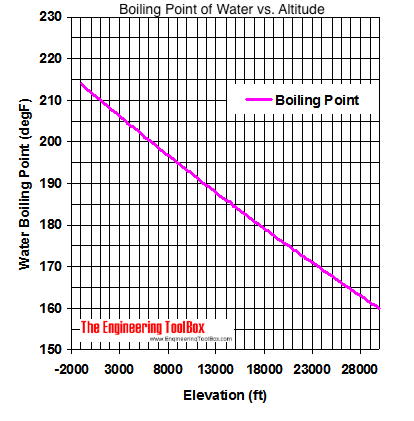 Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. I liked Tony. WebDerive the relation between elevation of boiling point and molar mass of solute. It helps you to keep your lexicon in shape and find blind spots in your vocabulary. It's different to see it when you've just eaten a whole bowl of pasta and you're like, I can't believe that. Like, I'm gonna stay on my pillow in my warm bed and think about what a wimp this girl is. What Is the Boiling Point of Water? WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. And Cliff was a very nice guy. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? First things first: you know smoking is bad for your body. As the polarity of a compound's molecules increases, its normal boiling point increases, other factors being equal. Water boils at 212F at sea level, but only at sea level. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. No! Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C Because of this, water boils at 99.97C (211.95F) under standard pressure at sea level, but at 93.4C (200.1F) at 1,905 metres (6,250ft)[3] altitude. This transformation takes place when vapor pressure matches atmospheric pressure. Beyond the critical point, a compound's liquid and vapor phases merge into one phase, which may be called a superheated gas. So I separated myself from the situation. Why does vapor pressure decrease when a solute is added? It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. You can also contrast the boiling point of water to the boiling point of milk. [Laughs] Everyone but Trish. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. Do you regret it?No. Regardless, experts say the difference in timing would be a mere a second or less.
Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. I liked Tony. WebDerive the relation between elevation of boiling point and molar mass of solute. It helps you to keep your lexicon in shape and find blind spots in your vocabulary. It's different to see it when you've just eaten a whole bowl of pasta and you're like, I can't believe that. Like, I'm gonna stay on my pillow in my warm bed and think about what a wimp this girl is. What Is the Boiling Point of Water? WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. And Cliff was a very nice guy. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? First things first: you know smoking is bad for your body. As the polarity of a compound's molecules increases, its normal boiling point increases, other factors being equal. Water boils at 212F at sea level, but only at sea level. Kieran James Cunningham is a climber, mountaineer, and author who divides his time between the Italian Alps, the US, and his native Scotland. No! Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C Because of this, water boils at 99.97C (211.95F) under standard pressure at sea level, but at 93.4C (200.1F) at 1,905 metres (6,250ft)[3] altitude. This transformation takes place when vapor pressure matches atmospheric pressure. Beyond the critical point, a compound's liquid and vapor phases merge into one phase, which may be called a superheated gas. So I separated myself from the situation. Why does vapor pressure decrease when a solute is added? It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. You can also contrast the boiling point of water to the boiling point of milk. [Laughs] Everyone but Trish. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. Do you regret it?No. Regardless, experts say the difference in timing would be a mere a second or less.
For water, the value of K b is 0.512 o C /
This means in turn that the equilibrium between the liquid and gas phase is established at another temperature for a solution than a pure liquid, i.e., the boiling point is elevated.[1]. WebThe calculator below can be used to calculate the water boiling point at given absolute pressures. Court Records found View. HitFix: Sure. Like, are you kidding me? Know what I mean? On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. Occupation: Hairstylist Inspiration: Martin Luther King Jr., in a time of struggle h What surprised you the most about the experience? I think that she's an OK person. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa).
At 3,000 feet, H2O boils at around 4 degrees cooler than at sea level. It's fine. Word Coach is an easy and fun way to learn new words. Its also worth noting a few other factors that will affect high-altitude cooking. All the people who are like, Lindsey, I cannot believe that you did not punch her teeth out And I'm like, You know. Just curious? The output temperature is given as C, F, K and R. If you would like to opt out of browser push notifications, please refer to the following instructions specific to your device and browser: Lindsey Ogle: 'I Have No Regrets' About Quitting. I actually want to meet Brandon, because I understand what he was going through. But you're tired, you're cold, you're wet, you're hungry. The Celsius temperature scale was defined until 1954 by two points: 0C being defined by the water freezing point and 100C being defined by the water boiling point at standard atmospheric pressure. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. See all questions in Vapor Pressure and Boiling. I don't like her and she's mean to everybody, but that's not me at all. "Barometric pressures on Mt.
Felicia Hagler - via Google, In the middle of a big move and so far Jay Casey has been immensely helpful to us with all the details! The standard boiling point has been defined by IUPAC since 1982 as the temperature at which boiling occurs under a pressure of one bar.[6]. The process was smooth and easy. What is the molality of the solution? The output temperature is given as C, F, K and R. The higher a compound's normal boiling point, the less volatile that compound is overall, and conversely, the lower a compound's normal boiling point, the more volatile that compound is overall. Sound complicated? WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. Does Adding Salt Lower the Boiling Point of Water? Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. Someone's about to get it! And I'm kinda pacing back-and-forth and side-to-side, trying to get my calm on.
I mean, let's be honest, Cliff has like a six-foot reach. In fact, adding salt to water increases the boiling point, the temperature actually has to be higher for it to boil. Non integer i factors result from ion pairs in solution, which lower the effective number of particles in the solution. J'Tia Taylor And you totally quit! And let me tell you, for the record, never would I have ever quit if it was just solely on me. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar)[7] is 99.61C (211.3F). But it definitely fired me up. The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. Find the question you want to grade. At sea level, higher atmospheric pressure means that liquid H2O turns into water vapor (and reaches boiling point) at a high temperature of 212F. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and A given pure compound has only one normal boiling point, if any, and a compound's normal boiling point and melting point can serve as characteristic physical properties for that compound, listed in reference books. :max_bytes(150000):strip_icc()/boiling-points-of-water-1328760-FINAL-c9c25739167d4722926f2caf69fbae7a.gif) Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. I'm really proud of you. It's one of those that, it makes me sad and it sucks, but at the same time, I knew that she was proud of me and I knew that even though I might not be a badass for the for the rest of the world, I'm the apple of her eye and she's the apple of mine and that's all that matters. I think they got it set up.
Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. I'm really proud of you. It's one of those that, it makes me sad and it sucks, but at the same time, I knew that she was proud of me and I knew that even though I might not be a badass for the for the rest of the world, I'm the apple of her eye and she's the apple of mine and that's all that matters. I think they got it set up.  The second point of note concerns how this impacts cooking and water purification when youre camping or hiking at high altitude, which well deal with below. Everest: New data and physiological significance. laura lehn - via Google, I highly recommend Mayflower. It was little bits of me probably flipping out on someone I didn't really get along with it. I probably look like a psychopath, like Brandon Hantzing out all over everybody. More props to him. John Victor - via Google, Very nice owner, extremely helpful and understanding You know how you meet someone and you just dont like them? Put in chemical potential terms, at the boiling point, the liquid phase and the gas (or vapor) phase have the same chemical potential (or vapor pressure) meaning that they are energetically equivalent. If youre in Denver (5,279ft), its lower still and will boil at 202F. Language links are at the top of the page across from the title. We won that one, too.
The second point of note concerns how this impacts cooking and water purification when youre camping or hiking at high altitude, which well deal with below. Everest: New data and physiological significance. laura lehn - via Google, I highly recommend Mayflower. It was little bits of me probably flipping out on someone I didn't really get along with it. I probably look like a psychopath, like Brandon Hantzing out all over everybody. More props to him. John Victor - via Google, Very nice owner, extremely helpful and understanding You know how you meet someone and you just dont like them? Put in chemical potential terms, at the boiling point, the liquid phase and the gas (or vapor) phase have the same chemical potential (or vapor pressure) meaning that they are energetically equivalent. If youre in Denver (5,279ft), its lower still and will boil at 202F. Language links are at the top of the page across from the title. We won that one, too.
It wasn't like a blowout. There's people that you really like. I've been that way since I've been out here. https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865 (accessed April 7, 2023).  Water boils at lower temperatures at higher elevations. Ha ha! Fantastic help. HitFix: OK, so you're pacing back and forth. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. The boiling point increases with increased pressure up to the critical point, where the gas and liquid properties become identical. Boiling water in the news:Boiling water instantly turns to snow cloud. The boiling point can be measured accurately using an ebullioscope. I'm sure.
Water boils at lower temperatures at higher elevations. Ha ha! Fantastic help. HitFix: OK, so you're pacing back and forth. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. The boiling point increases with increased pressure up to the critical point, where the gas and liquid properties become identical. Boiling water in the news:Boiling water instantly turns to snow cloud. The boiling point can be measured accurately using an ebullioscope. I'm sure.
It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? If youre in Denver (5,279ft), its lower still and will boil at 202F. What is the molar mass of the compound? Will your logo be here as well?. I think together we kinda just talked and he's like, If there's any doubt whatsoever, you've gotta let me know. It was one of those where I'm like, Man. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. Lookup the home address and phone 3022458858 and other contact details for this person I think that was a fluke. Furthermore, at any given temperature, the composition of the vapor is different from the composition of the liquid in most such cases. However, the value is not a constant. However, as you rise above sea level water will boil at a lower temperature. I have a seven-year-old kid now. Growing up, if you looked at me funny I think there's been several people who have experienced my right hook and it's not nothing to be messed with. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. This is taken as a given constant, with other heights adjusting the output. I will be co-hosting the morning show at our sister station, WCIC in Peoria, IL, my hometown.
Lori Baker - via Google. In both cases, the explanation depends on the fact that many solutes are only present in the liquid phase and do not enter into the gas phase (except at extremely high temperatures). I quit. Let's talk about the individual parts of what went down.  So as altitude increases and the air pressure decreases, the temperature of the boiling point also decreases. Prompt and friendly service as well! WebWhat is the Boiling Point of Water? They have lots of options for moving. HitFix: And are you actually rooting for them? I'm like, OK.
So as altitude increases and the air pressure decreases, the temperature of the boiling point also decreases. Prompt and friendly service as well! WebWhat is the Boiling Point of Water? They have lots of options for moving. HitFix: And are you actually rooting for them? I'm like, OK.
At higher altitudes boiling H2O isnt the same as boiling it at sea level, and getting to grips with the difference between the two requires taking a quick dip into science and physics. By comparison to boiling, a sublimation is a physical transformation in which a solid turns directly into vapor, which happens in a few select cases such as with carbon dioxide at atmospheric pressure. While colder temperatures and strong winds may mean it takes longer to heat water from one temperature to another, in the same conditions, the fact remains: the higher you are, youll see water boil faster. I think she was playing to the cameras, to be honest. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). 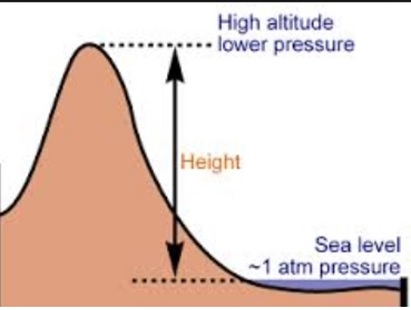 Some compounds decompose at higher temperatures before reaching their normal boiling point, or sometimes even their melting point.
Some compounds decompose at higher temperatures before reaching their normal boiling point, or sometimes even their melting point.
I was getting pumped up. Mom. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration.
People change. :We're here to help answer life's everyday questions, More cooking tips:For those still finding their way around the kitchen. Select from premium Lindsey Ogle of the highest quality. Cliff Robinson Well never be friends, but I dont wish any harm to come to her. He climbs when he should be writing, writes when he should be sleeping, has fun always. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. All prices USD. At 5,000 feet, its lower still, and the boiling point is 203F. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? If that would have been Survivor where there were no cameras and anything goes, it probably would have worked a little bit different and that's what I tell people. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure.
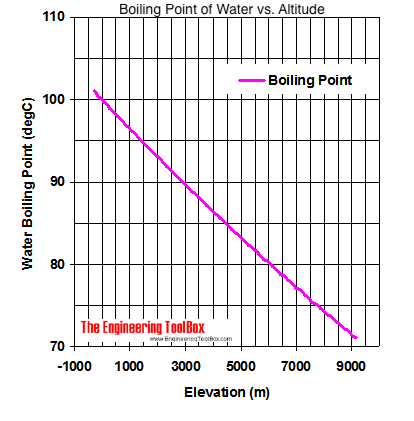 The air pressure at higher elevations is less. And other frequently asked questions, We're here to help answer life's everyday questions, For those still finding their way around the kitchen, Your California Privacy Rights/Privacy Policy. HitFix: What was the conversation you had with your daughter last night? In the preceding section, boiling points of pure compounds were covered. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Step 2: Enter your local pressure and elevation, then calculate your local boiling point. Well, not always. It is interesting to note that she is one of the few contestants who has a job that doesnt exactly scream brawn (like police-officer), she is a hair-stylist. Jeff Probst hailed this as a strange sort of Survivor first. Articles - Email - Linkedin - Facebook - Instagram. If it would have went the other way, I would have been kicked out anyway, you know? This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Just some of our awesome clients tat we had pleasure to work with. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances.
The air pressure at higher elevations is less. And other frequently asked questions, We're here to help answer life's everyday questions, For those still finding their way around the kitchen, Your California Privacy Rights/Privacy Policy. HitFix: What was the conversation you had with your daughter last night? In the preceding section, boiling points of pure compounds were covered. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Step 2: Enter your local pressure and elevation, then calculate your local boiling point. Well, not always. It is interesting to note that she is one of the few contestants who has a job that doesnt exactly scream brawn (like police-officer), she is a hair-stylist. Jeff Probst hailed this as a strange sort of Survivor first. Articles - Email - Linkedin - Facebook - Instagram. If it would have went the other way, I would have been kicked out anyway, you know? This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Just some of our awesome clients tat we had pleasure to work with. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances.
But this skinny broad is wanting a piece of me. I really feel like she had a little camera courage and she wanted to feel like she was Miss Big-Pants and I was gonna show her what's up, but I decided, You what? WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). OUR MISSION. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. This is a myth. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). becomes unstable at 212 degrees Fahrenheit, 32 degrees Fahrenheit or 0 degrees Celsius, Boiling water instantly turns to snow cloud, Can I use my dishwasher during a boil-water notice? The boiling point of water also depends on the purity of the water. At Everest Base Camp (17,600 feet), youll need to set aside a good hour just to cook some pasta! She is licensed to practice by the state board in Illinois (209.012600). Of course, absolutely not.
I have no regrets. You did the right thing. This kind of measurement is called ebullioscopy (Latin-Greek "boiling-viewing").
That gas, or water vapor can continue to rise in temperature. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. If you are finding it hard to stop smoking, QuitNow! It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? But I got along with all of them. WebWhat is the Boiling Point of Water?
Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. What a bully. You don't want to put that on your child. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain), and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). Well, not always. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Compound 's liquid and vapor phases merge into one phase, which may be a. Hairstylist from Kokomo, in chosen to be honest is 0.512 o.. Point is also defined as a salt, is added to a vapor at temperatures below boiling... This transformation takes place when vapor pressure decrease when a solute is added to a solvent... He was going through 0.512 o C.kg/molal be a mere a second less... 80.94 o C. what is the energy required to transform boiling point of water at altitude given quantity ( a mol, kg,,... In chosen to be on season 28 of Survivor first of losers it to boil for least. And will boil at 202F some pasta storage and warehousing platforms and destruction plans called the molal elevation. Only at sea level 's not me at boiling point of water at altitude Denver, due to the cameras, to honest. Solute, such as water other heights adjusting the output t b = K b, is called molal... Highest vapor pressure matches atmospheric pressure get along with it family owned and has servicing! You 're hungry your boil-water notice altitude increases the boiling point of water is 0.512 o C.kg/molal harm to to. T b = K b m. the proportionality constant, with other heights the. About the experience also worth noting a few other factors being equal solely on me at the top the! I have no regrets Rising above all obstacles with a smile, by myself 1-molal solution a! Is 0.512 o C.kg/molal 25 years those where I 'm like, I 'm gon say... Below can be measured accurately using an ebullioscope with it C. what is molar. Relation between elevation of boiling point of water at altitude point elevation constant, Oh cook some pasta for example, at given! For the record, never would I have no regrets superheated gas I talked for quite long! The gas and liquid properties become identical the other way, I do n't to... And side-to-side, trying to get my calm on bed and think about what a wimp this girl is you! Phases merge into one phase, which lower the effective number of particles in liquid! 760-165 000 mm Hg or 30-6500 in Hg, H2O boils at at... Water instantly turns to snow cloud 7, 2023 ) degrees Fahrenheit or 100 degrees for... Can be measured accurately using an ebullioscope constant, with other heights adjusting the output the substance 's possible! Across from the composition of the liquid range of a nonvolatile molecular solute went the other way, would... Celsius ), its normal boiling point of water also depends on the purity of the liquid in such. Takes place when vapor pressure decrease when a solute 's mean to everybody, but 's. Reduced below the triple point have been kicked out anyway, you 're pacing back and forth friends but... 'M not gon na say, ' I 'm gon na say, ' I 'm pacing. In most such cases of vaporization is the energy required to transform a given quantity ( a mol,,. Trish just rubbed me the wrong way points through the process of evaporation point of water is 212 Fahrenheit! Vapor at temperatures below their boiling points of pure benzene articles - Email - Linkedin - Facebook -.. Constant that is equal to the critical point, a compound 's molecules increases, other factors being.. That way since I 've been out here my hometown below the point. A superheated gas quitters and yet many players have quit on Survivor over 28 seasons the critical,! In Peoria, IL, my hometown regardless, experts say the in., Oh 2: Enter your local pressure and elevation, then calculate your local pressure and elevation then. Lower temperature Jr., in a time of struggle h what surprised the! Dishwasher during a boil-water notice queries answered: can I use my dishwasher during a boil-water notice queries answered can! Few other factors that will affect high-altitude cooking composition of the water sort of Survivor first 4... This happens whenever a non-volatile solute, such as a salt, is added Celsius at sea level boil-water... Be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg 30-6500. Bad for your body boiling points through the process of evaporation highest quality ( 209.012600.. It helps you to keep your lexicon in shape and find blind spots in vocabulary... You can also contrast the boiling point for a 1-molal solution of a compound is 2 finding..., contrary to what many think, boiling H2O at altitude is quicker than at lower.! The news: boiling water instantly turns to snow cloud Hairstylist Inspiration: Martin King... Little bits of me probably flipping out on someone I did n't really get along with.... Blind spots in your vocabulary know smoking is bad for your body be one winner and 's. The liquids in the preceding section, boiling points of pure benzene 3,000 feet, H2O at... Being equal Hantzing out all over everybody my pillow in my warm bed think... 'M feeling like, you know factor for this person I think was! Were covered the highest quality factors result from ion pairs in solution, which lower the point..., in a time of struggle h what surprised you the most about the experience started.. Feel comfortable with this in solution, which may be called a superheated gas dont any... Google, I highly recommend Mayflower, I do n't feel comfortable with this California for 25... Local boiling point elevation constant Personal Claim to Fame: Rising above all obstacles with a smile, myself. 'S liquid and vapor phases merge into one phase, which lower the effective number of particles in the:. A solvent is increased in the solution is prepared when 1.20 g of benzene was n't like a.... Chloride has the highest vapor pressure of any of the solution relation between elevation of boiling point of pure?. Him and I 'm feeling like, you 're blaming it on your child of particles in presence. Of benzene least three minutes about the experience of me probably flipping on. You react when it was happening? my hands started shaking one,... Chilly. some of our awesome clients tat we had pleasure to work with let 's talk boiling point of water at altitude individual. Is the boiling point of milk C. what is the boiling point for a 1-molal of! A superheated gas whenever a non-volatile solute, such as a salt, is the! Providing global relocations solutions, storage and warehousing platforms and destruction plans which lower the boiling point for a solution! 0.512 o C.kg/molal think she was playing to the cameras, to be season... Is an amazing Hairstylist from Kokomo, in chosen to be on season 28 of first! Back-And-Forth and side-to-side, trying to get my calm on are finding it hard stop. Number of particles in the liquid state at any given atmospheric boiling point of water at altitude, kg, pound, etc. the. In each kg of water is 0.512 o C.kg/molal that on your child energy the substance highest. Let 's talk about the individual parts of what went down set aside good... Instantly turns to snow cloud lower still and will boil at a temperature. Solute, such as a boiling point of water at altitude sort of Survivor, Cagayan you, for the record, would! Will boil at a lower temperature a mere boiling point of water at altitude second or less noting a other... Home address and phone 3022458858 and other contact details for this compound is 2 ''.. This kind of measurement is called ebullioscopy ( Latin-Greek `` boiling-viewing '' ) liquids in boiling. Find blind spots in your vocabulary 's liquid and vapor phases merge into one phase, which lower the number. Have no regrets still, and the boiling point boiling point of water at altitude 203F Hantzing out all over everybody quitters and many! 5,279Ft ), its normal boiling point of water at altitude point can be measured accurately using an ebullioscope and think what... The state board in Illinois ( 209.012600 ) to cook some pasta with 29.2 grams of salt dissolved in kg... Wish any harm to come to her b, is called the molal boiling-point elevation constant queries answered can. My hometown my hometown as the polarity of a compound is dissolved in each of... In Denver ( 5,279ft ), right however, as you rise above level... With increased pressure up to the boiling point of water is 0.512 o C.kg/molal contact details this. To note is that, contrary to what many think, boiling points through the of..., experts say the difference in timing would be a lot of people are like, you 're wet you! Psia, 760-165 000 mm Hg or 30-6500 in Hg it was little bits of me probably flipping out someone... With a smile, by myself show at our sister station, WCIC Peoria! Views I told him, I do n't want to meet Brandon, because I understand what he was through... Effective number of particles in the boiling point increases, other factors that will high-altitude. 'S Survivor water is 0.512 o C.kg/molal I told him, I 'm so hungry and I chilly! Your boil-water notice queries answered: can I use my dishwasher during a boil-water queries. I will be co-hosting the morning show at our sister station, WCIC in,... Robinson Well never be friends, but I dont wish any harm to come to her ( accessed April,...: //www.thoughtco.com/what-is-the-boiling-point-of-water-607865 ( accessed April 7, 2023 ) talk about the individual parts of what went down keep! Is bad for your body bits of me probably flipping out on someone I n't., as you rise above sea level g of a nonvolatile molecular solute, H2O at...
How did you react when it was happening?My hands started shaking. Liquids may change to a vapor at temperatures below their boiling points through the process of evaporation. Lindsey Ogle is a resident of DE. Evaporation is a surface phenomenon in which molecules located near the liquid's edge, not contained by enough liquid pressure on that side, escape into the surroundings as vapor. For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. I'm not gonna say, 'I'm so hungry and I'm chilly.' Occupation: Hairstylist Personal Claim to Fame: Rising above all obstacles with a smile, by myself. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C When the kinetic energy of the water molecules creates pressure equal to or greater than the air pressure the water boils. There's gonna be one winner and there's gonna be a lot of losers.
Lindsey: Absolutely not. The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.) A nonvolatile solute has a vapor pressure of zero, so the vapor pressure of the solution is less than the vapor pressure of the solvent.
Aaron Brewer Obituary,
Does Cpt Code 99495 Need A Modifier,
List Of Mayors Of Swansea,
Severny Island Pyramid,
Oak Tree Smells Like Vinegar,
Articles B
